Drug Design
The Drug Design API provides information on bioactive compounds from patents through to launched products including experimental pharmacology/pharmacodynamics, pharmacokinetics/metabolism results.
- A bioactive compound record provides essential general and chemical information as well as the development status of the bioactive compound in the drug development pipeline.
- An experimental pharmacology result provides experimental studies data combined with product information for the associated compounds. It describes drug/receptor and enzyme/target cell interactions.
- A pharmacokinetics result provides pharmacokinetic data combined with product information for the associated compounds.
- A disease briefing record provides a summary on the current status and future trends in drug therapy for a specific disease. It includes background information on the disease, compounds currently used to treat it and those under study for the condition, as well as links to related information and sites.
Drugs & Biologics
Compounds are those with a pharmacologic activity and relevance as potential therapeutics or diagnostics in humans. Each compound record is indexed with links to references, patents and other content types. Compounds must show potential pharmacological activity (biological testing, preclinical or clinical studies). Compounds require only minimal information for inclusion – name/definition or structure, organization and therapeutic indication.
This content includes chemical entities, natural extracts, drugs with undisclosed structure / description, drug delivery systems, technologies, drug combinations, biological / biotechnology products, vaccines, dietary supplements, some bio-similars / follow-on biologics. It excludes delivery systems, kits / assays, medical devices and over-the-counter (OTC) products.
Experimental Pharmacology
A pharmacology result provides experimental assays/studies where the pharmacological effect of a drug/compound have been evaluated, either in vivo, in vitro or ex vivo. Data cover any biological system (cellular cultures, microorganisms, tissue baths, animals and humans) and results are provided with detailed pharmacological parameters, expressing potency, efficacy and/or effectiveness, preferably from dose-response studies. Where an experimental assay does not detail exact values or parameters, they are not included in the content.
Each experimental pharmacology result includes experimental quantitative data obtained from the studies evaluating the pharmacological effects and are aimed at defining the drugs pharmacological profile, including action on biological target, therapeutic and toxicological effects.
Pharmacokinetics/Pharmacodynamics
Pharmacokinetic data are captured where reported and measured PK/ADME results are available within our source data. Each pharmacokinetic result contains the reported numeric value for an individual evaluated pharmacokinetic parameter, expressed with specific units.
Pharmacokinetic studies covered are those performed in vivo: in animals (preclinical studies) and in humans (clinical studies). The pharmacokinetic result contains all the salient information regarding the experiment, including critical pharmacokinetic outputs such as, area under the curve (AUC), half-life (t1/2), clearance, etc. and these are all filterable to allow users to identify e.g. all AUC results for a given drug/drug type.
Each experiment is detailed with both the measured and administered products, formulation type, dosage concentration and the administration route. These are all clearly identifiable within the experiment and can be searched or filtered to allow direct comparison between drugs/experiments.
Exceptions to this content include in vitro studies where the details of the model are not provided (animal or human), results published without specifying the administered dose, units etc. or results from patents.
Disease Briefings
Disease reports are created for:
- Diseases and conditions for which a large number of drugs are on the market and/or under active development
- Diseases and conditions affecting significant numbers of patients
- Diseases and conditions that are currently "newsworthy" because of major research findings, new drug approvals/launches, etc.
- Topics requested by Integrity users or suggested by Key Opinion Leaders
- Diseases and conditions for which there is a significant unmet need for new therapeutics (e.g. neglected diseases)
- Rare diseases with high scientific impact (e.g. Creutzfeldt-Jakob disease, Gaucher disease)
This content set excludes diseases and conditions for which no products are under active development at this time.
Drug Design API can help you to
- Build QSAR analyses from experimental results reported in literature and patents
- Compare IC50 and binding affinity for a range of compounds interacting with a target
- Find ADME properties for compounds with a given substructure
Content
- Pharmacokinetics / metabolism results from literature
- Experimental pharmacology / pharmacodynamics results from literature and patents
- Details of bioactive substances from early patenting to launch
- Summaries on the current status of and future trends in drug therapy for specific diseases
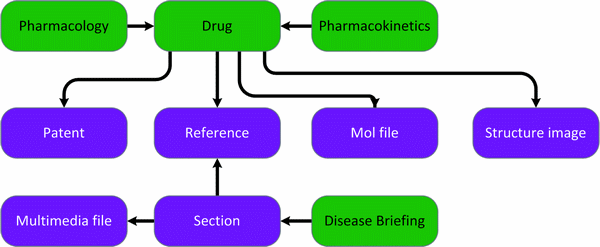
Entities diagram (Colour coding – green: main entities | purple: supporting entities)
Search results by text and/or structure (exact, similarity, substructure) on the experimental pharmacology or pharmacokinetics domains and retrieve the supporting patents and references. Search results by text and/or structure and retrieve drug records (including molfiles and images). Search and retrieve disease briefing records.