Clinical
The Clinical API provides access to manually curated information on worldwide clinical trials from 24 major trial registries (covering drugs, biologics, devices and biomarkers), press releases (from 1987 to date), conference abstracts and reports (from 2007 to date). It includes access to clinical literature (from 1964 to date – structure searchable) based on Derwent Drug File (DDF) data.
Each entry provides a protocol report, an outcomes report (where appropriate) and all associated links to drugs (for both the interventions & controls in the trial), drug classes and actions, companies (sponsors and collaborators of the trial), inclusion and exclusion criteria, supporting references (including reports from conferences and abstracts), and other trial records where there are specific trial-trial relationships. Please see the Clinical API 2.0-FAQ for more details.
Clinical API can help you to
- Stay up to date with the latest clinical trial information and get to the data quickly
- Be confident the results returned reveal the most up-to-date trial outcomes and adverse event information
- Inform your trial design by obtaining completive intelligence about other trials, what sponsors are running them, how they are designed, what drugs are used
- Perform cross trial analysis using drugs used and their mechanisms, biomarkers, outcomes and adverse events
- Match complete company, drug and biomarker information from other Cortellis APIs to inform and broaden your analysis
- Match patients to trials programmatically using drugs, recruitment status, indication, gene variants, inclusion and exclusion criteria
Content
- Clinical trial content from international registry sources
- Registries received through feed: CT.gov, EudraCT (EU), UMIN (Japan), ISRCTN (global), ANZCTR (Australia, New Zealand), IRCT (Iran), NTR (Netherlands), HKCT (Hong Kong CTR) and DRKS (Germany)
- Registries captured manually: ChiCTR (China), JapicCTI (Japan), CTRI (India), CRiS (Korea), NMRR (Malaysia), HSA CTR (Singapore), JMACCT CTR (Japan), ReBec (Brazil), PHRR (Philippines), TCTR (Thailand), SRM CTR (Russia), Mexico CTR (Mexico), SLCTR (Sri Lanka), PACTR (Pan African), RPCEC (Cuba) and UKCRN (UK – coverage halted as of late 2016)
- Summaries of selected journal articles (DDF)
- Trial outcomes derived from journals, conferences and press releases
- Pharmaceutical company press releases and information from major conferences
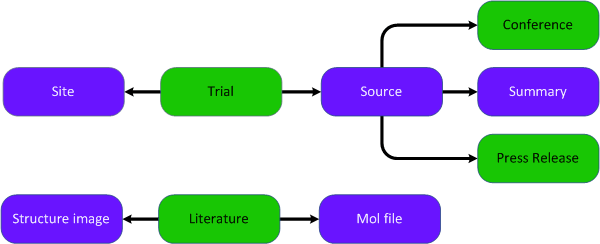
Entities diagram (Colour coding – green: main entities | purple: supporting entities)
Search results, retrieve and export trial (including sites), press release, conference and clinical literature records. Search and browse different controlled vocabularies (endpoints, patient segment, exclusion criteria, inclusion criteria, companiesSponsor, companiesCollaborator, trialCompanies, category).