Biomarkers
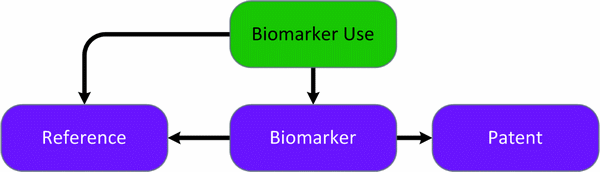
The Biomarkers API provides a wealth of reliable, high-quality, continually updated information supporting biomarker research at every stage of the drug R&D process. The underlying database is the first one to standardize terminology and classify biomarkers reliably into lifecycle phases and disciplines. It helps improve cost efficiency, predict and minimize risk and avoid late stage attrition. The Biomarker Use data are supported by Biomarker, Patent and Reference records.
Biomarkers API can help you to
- Understand the biology of the disease you’re working on, as well as the research status for biomarkers in your therapy area
- Choose the biomarker that will provide early indications of the impact of target modification on the disease process
- Find the biomarkers that show the effects of your compound on the disease state
- Select suitable biomarkers for use in safety and efficacy studies to give early indication of toxicity and proof of concept
- Substantiate claims and develop clinical guidelines by consulting a body of carefully curated biomarker information
- Determine the population that will respond best to the particular treatment
- Identify biomarkers to advance the design and efficacy of your studies and improve your probability of success
- Evaluate evidence (both supporting and refuting) to assess the relative merits of a biomarker for your own research
- Find validated biomarkers for clinical trials and cross-reference with the clinical API to identify stratified populations
Content
- Individual records for each distinct use:
- Biomarker name, role
- Indication and its type
- Population
- Technique used to measure the biomarker
- Substrate used for the measurement technique
- Drug / group of drugs
- Supporting references, patents
- Biomarker summaries
- Biomarker name, synonyms, external data source identifiers (UniProtKB, GenBank, PDB, EntrezGene, KEGG), type, description
- Biological process
- Supporting references, patents
- All reference or patents sources along with their evidence types (Supporting, Neutral, Conflicting)
- Reference title, authors, source, year, volume, issue, page, PubMedID
- Conference name, location, start/end date, edition
- Patent publication number, publication date, title, inventors, applicants
Entities diagram (Colour coding – green: main entity | purple: supporting entities)
Search biomarker use results and retrieve biomarkers use, biomarker, patent and reference records.